For organizations, businesses, and stakeholders in the food supply chain, the case for robust food traceability capabilities has never been stronger. Not only has the global food supply chain become incredibly complex and vast – recent years have highlighted its fragility and warranted a stronger approach to managing the inherent risks. With the passing of Section 204(d) of the Food Safety Modernization Act (FSMA 204), suppliers, retailers, wholesalers, and more will be held to more stringent standards around tech-enabled traceability.
Traceability: Then and Now
Long before FSMA 204, the term “traceability” first appeared in 1996 in the international ISO 8402 standard concerning quality management and quality assurance; its origins stretch well beyond the last 20th century. In fact, the earliest known records documenting the practice of tracing the origin of a disease from a food product stretch back to 1275 from a written account by Thomas of Walsingham concerning the infection of an ewe that led to an outbreak of disease impacting England for nearly 28 years. Needless to say, the roots of traceability principles have been a part of our food supply chain for quite some time.
Over time, the approach to traceability in the food ecosystem has (thankfully) evolved. Fast forward to our present day, existing FDA regulations employ the “one step forward, one step back” approach. In this approach, food businesses must be able to identify the companies “one step forward,” i.e., who have received their product, and those “one step back,” i.e., who immediately supplied products or ingredients to them. While a step in the right direction, this approach has left blind spots in the food ecosystem and prevented further optimization of food recalls and recall processes.
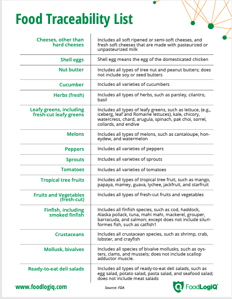 With FSMA 204, the FDA is poised to address the broad swaths of the food supply chain that have been left out of previous regulatory actions. The new regulation will require full traceability and will also mandate robust recordkeeping and easily transmissible data to enable more effective and rapid recall management. That said, the scope is currently limited to a small subset of products deemed high-risk by the FDA, all found on the Food Traceability List. For companies carrying these products, the imminent need for a traceability solution is clear. It may seem like a pass for companies with products or ingredients outside of the FTL, but this is just the beginning of the move towards tech-enabled traceability of the entire food supply chain - a move the FDA has clearly indicated as the ultimate goal.
With FSMA 204, the FDA is poised to address the broad swaths of the food supply chain that have been left out of previous regulatory actions. The new regulation will require full traceability and will also mandate robust recordkeeping and easily transmissible data to enable more effective and rapid recall management. That said, the scope is currently limited to a small subset of products deemed high-risk by the FDA, all found on the Food Traceability List. For companies carrying these products, the imminent need for a traceability solution is clear. It may seem like a pass for companies with products or ingredients outside of the FTL, but this is just the beginning of the move towards tech-enabled traceability of the entire food supply chain - a move the FDA has clearly indicated as the ultimate goal.
Meeting Regulatory Demands with Tech-Enabled Traceability
In order to meet the traceability regulations set forth by FSMA 204, companies are pursuing a wide variety of technological and strategic solutions. While it may seem appealing to cobble together a solution across various existing technologies or modular add-ons, establishing a centralized, single-source-of-truth platform should be the ultimate objective for food businesses. In doing so, you can enable capabilities that both meet regulatory guidelines and create a foundation for sustainable and effective food safety, supply chain resilience, and business growth.
Tech-enabled traceability solutions like FoodLogiQ’s Track + Trace enable wide-ranging benefits to your business, stretching across functional areas and including, but not limited to:
- End-to-end supply chain visibility
- Robust and user-friendly data reporting, analytics, and dashboarding
- Centralized, effective communication and collaboration with supply chain partners
- Readily accessible, data-backed transparency and visibility
FoodLogiQ’s solutions allow for real-time digital management of the Key Data Elements (KDEs) and Critical Tracking Events (CTEs) necessary to meet FSMA 204 compliance. Beyond that, our traceability solutions are rooted in GS1 standards and aligned with the FDA’s approach to enhanced traceability recordkeeping. Regardless of where your business is in your journey to establishing a traceability solution, FoodLogiQ can help you along the way to ensure effective implementation of the system within your business, adoption into your business processes, and onboarding of your entire network of suppliers and supply chain partners.
While the complexities of the global supply chain have warranted the need for tech-enabled traceability, the same complexities have made it all the more difficult to unify the various stakeholders in a single business’ operations into a traceability solution. FoodLogiQ’s solutions are employed by industry leaders across different food industry sectors, including retailers, suppliers, wholesalers, and more.
Other posts you might be interested in
View All Posts
Industry Regulations
10 min read
| February 10, 2023
2023 Top Consumer Supplement Trends
Read More
Trustwell News
4 min read
| April 13, 2023
Salad And Go Turns to Trustwell’s FoodLogiQ Solution for Growth, Transparency, and Trust
Read More
Food Industry
5 min read
| February 1, 2023

